


Next: The Time-Dependent Schrödinger Equation
Up: The Schrödinger Equation
Previous: The Schrödinger Equation
Here we follow the treatment of McQuarrie [1], Section
3-1. We start with the one-dimensional classical wave equation,
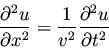 |
(10) |
By introducing the separation of variables
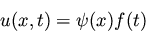 |
(11) |
we obtain
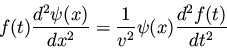 |
(12) |
If we introduce one of the standard wave equation solutions for f(t)such as
 (the constant can be taken care of later in the
normalization), we obtain
(the constant can be taken care of later in the
normalization), we obtain
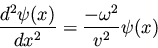 |
(13) |
Now we have an ordinary differential equation describing the spatial
amplitude of the matter wave as a function of position. The energy
of a particle is the sum of kinetic and potential parts
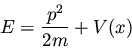 |
(14) |
which can be solved for the momentum, p, to obtain
![\begin{displaymath}p = \{ 2m [ E - V(x) ] \} ^{1/2}
\end{displaymath}](/Quantum%20Mechanics/Tutorial/notes/quantum_doc/img23.png) |
(15) |
Now we can use the de Broglie formula (4) to get
an expression for the wavelength
![\begin{displaymath}\lambda = \frac{h}{p} = \frac{h}{\{ 2m [ E - V(x) ] \}^{1/2}}
\end{displaymath}](/Quantum%20Mechanics/Tutorial/notes/quantum_doc/img24.png) |
(16) |
The term
 in equation (13) can be rewritten
in terms of
in equation (13) can be rewritten
in terms of  if we recall that
if we recall that
 and
and
 .
.
![\begin{displaymath}\frac{\omega^2}{v^2} = \frac{4 \pi^2 \nu^2}{v^2} =
\frac{4\pi^2}{\lambda^2} = \frac{2m[E - V(x)]}{\hbar^2}
\end{displaymath}](/Quantum%20Mechanics/Tutorial/notes/quantum_doc/img29.png) |
(17) |
When this result is substituted into equation (13) we
obtain the famous time-independent Schrödinger equation
![\begin{displaymath}\frac{d^2\psi(x)}{dx^2} + \frac{2m}{\hbar^2} [ E - V(x)]\psi(x) = 0
\end{displaymath}](/Quantum%20Mechanics/Tutorial/notes/quantum_doc/img30.png) |
(18) |
which is almost always written in the form
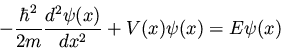 |
(19) |
This single-particle one-dimensional equation can easily be extended to
the case of three dimensions, where it becomes
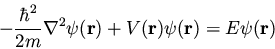 |
(20) |
A two-body problem can also be treated by this equation if the mass
m is replaced with a reduced mass  .
.
It is important to point out that this analogy with the classical wave
equation only goes so far. We cannot, for instance, derive the
time-dependent Schrödinger equation in an analogous fashion
(for instance, that equation involves the partial first derivative
with respect to time instead of the partial second derivative). In
fact, Schrödinger presented his time-independent equation first, and
then went back and postulated the more general time-dependent
equation.



Next: The Time-Dependent Schrödinger Equation
Up: The Schrödinger Equation
Previous: The Schrödinger Equation
![\begin{displaymath}\lambda = \frac{h}{p} = \frac{h}{\{ 2m [ E - V(x) ] \}^{1/2}}
\end{displaymath}](/Quantum%20Mechanics/Tutorial/notes/quantum_doc/img24.png)